Power from Sunlight – Powering space exploration with solar energy
Brief description: In this set of activities, students will learn about two concepts that influence solar panel design for space missions: the inverse square law

 Brief description:
Brief description:
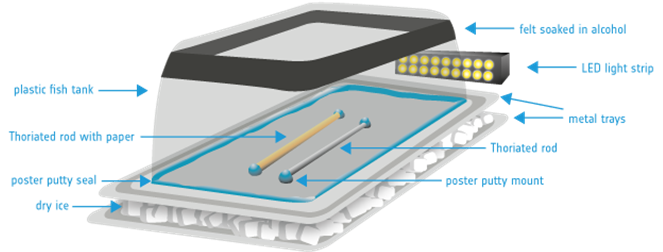

Cassini-Huygens at Saturn
Keywords:

Brief description: In this set of activities, students will learn about two concepts that influence solar panel design for space missions: the inverse square law
Brief description: In this activity, students will use an elliptical board to obtain speed and distance measurements for an object in an elliptical orbit. The
Brief description: In this activity, the principle of moments is applied to rotating systems to demonstrate the concept of a barycentre, or centre of mass,